Content deleted Content added
Tags: Reverted Visual edit |
|||
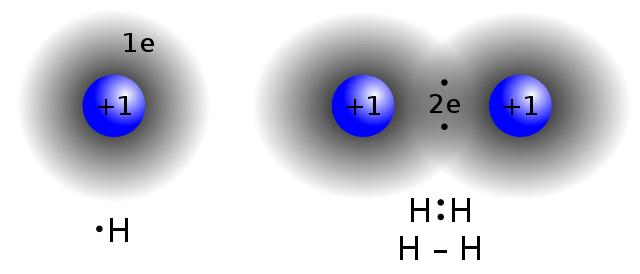
Line 27: == Covalent structures == There are several types of structures for covalent substances, including individual molecules, [[molecular structures]], [[macromolecular]] structures and giant covalent structures. Individual molecules have strong bonds that hold the atoms together, but generally, there are negligible forces of attraction between molecules. Such covalent substances are usually gases, for example, [[HCl]], [[Sulfur dioxide|SO<sub>2</sub>]], [[Carbon dioxide|CO<sub>2</sub>]], and [[Methane|CH<sub>4</sub>]]. In molecular structures, there are weak forces of attraction. Such covalent substances are low-boiling-temperature liquids (such as [[ethanol]]), and low-melting-temperature solids (such as [[iodine]] and solid CO<sub>2</sub>). Macromolecular structures have large numbers of atoms linked by covalent bonds in chains, including synthetic polymers such as [[polyethylene]] and [[nylon]], and biopolymers such as [[protein]]s and [[starch]]. [[Network covalent bonding|Network covalent structures]] (or giant covalent structures) contain large numbers of atoms linked in sheets (such as [[graphite]]), or 3-dimensional structures (such as [[diamond]] and [[quartz]]). These substances have high melting and boiling points, are frequently brittle, and tend to have high electrical [[resistivity]]. Elements that have high [[electronegativity]], and the ability to form three or four electron pair bonds, often form such large macromolecular structures.<ref name="StranksEtAl1970">{{cite book |last1=Stranks |first1=D. R. |last2=Heffernan |first2=M. L. |last3=Lee Dow |first3=K. C. |last4=McTigue |first4=P. T. |last5=Withers |first5=G. R. A. |title=Chemistry: A structural view |year=1970 |publisher=Melbourne University Press |location=[[Carlton, Victoria|Carlton, Vic.]] |isbn=0-522-83988-6 |page=184}}</ref>▼
▲[[Network covalent bonding|Network covalent structures]] (or giant covalent structures) contain large numbers of atoms linked in sheets (such as [[graphite]]), or 3-dimensional structures (such as [[diamond]] and [[quartz]]). These substances have high melting and boiling points, are frequently brittle, and tend to have high electrical [[resistivity]]. Elements that have high [[electronegativity]], and the ability to form three or four electron pair bonds, often form such large macromolecular structures.<ref name="StranksEtAl1970">{{cite book |last1=Stranks |first1=D. R. |last2=Heffernan |first2=M. L. |last3=Lee Dow |first3=K. C. |last4=McTigue |first4=P. T. |last5=Withers |first5=G. R. A. |title=Chemistry: A structural view |year=1970 |publisher=Melbourne University Press |location=[[Carlton, Victoria|Carlton, Vic.]] |isbn=0-522-83988-6 |page=184}}</ref> == One- and three-electron bonds == | |||
 Article Images
Article Images