Glycerol: Difference between revisions - Wikipedia
 Article Images
Article Images
Line 1:
{{short description|Chemical compound widely used in food and pharmaceuticals}}{{Redirect|Glycerine|the song by Bush|Glycerine (song)}}{{Use dmy dates|date=SeptemberAugust 20202024}}
{{Chembox
| Watchedfields = changed
Line 13:
| ImageName3 = Sample of glycerine
| PIN = Propane-1,2,3-triol<ref>{{cite book |author=[[International Union of Pure and Applied Chemistry]] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=[[Royal Society of Chemistry|The Royal Society of Chemistry]] |pages=690 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref>
| OtherNames = {{Unbulleted list
| Glycerin<br
| />Glycerine<br
| />1,2,3-Trioxypropane<br
| />1,2,3-Trihydroxypropane<br
| />1,2,3-Propanetriol
}}
| Section1 = {{Chembox Identifiers
|IUPHAR_ligand = 5195
Line 35 ⟶ 41:
|DrugBank_Ref = {{drugbankcite|correct|drugbank}}
|DrugBank = DB04077
|ChEBI = 1752217754
|ChEBI_Ref = {{ebicite|correct|EBI}}
|SMILES = OCC(O)CO
Line 61 ⟶ 67:
| Section4 = {{Chembox Hazards
|ExternalSDS = [https://web.archive.org/web/20110505085357/https://www.jtbaker.com/msds/englishhtml/g4774.htm JT Baker ver. 2008 archive]
|NFPA-H = 01
|NFPA-F = 1
|NFPA-R = 0
Line 71 ⟶ 77:
}}
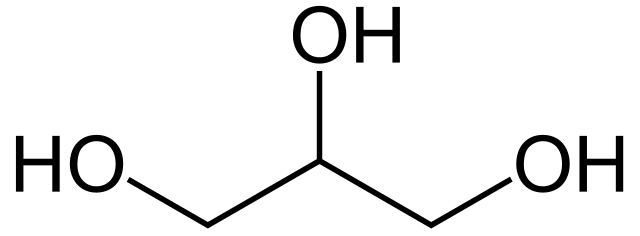
'''Glycerol''' ({{IPAc-en|ˈ|g|l|ɪ|s|ə|r|ɒ|l}}),<ref>{{cite web|url=https://www.oxforddictionaries.com/definition/english/glycerol|title=glycerol – Definition of glycerol in English by Oxford Dictionaries|website=Oxford Dictionaries – English|access-date=21 February 2022|archive-date=21 June 2016|archive-url=https://web.archive.org/web/20160621205507/http://www.oxforddictionaries.com/definition/english/glycerol|url-status=dead}}</ref> also called '''glycerine''' or '''glycerin''', is a simple [[triol]] compound. It is a colorless, odorless, [[viscous]] liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in [[lipid]]s known as [[glyceride]]s. Because it has [[antimicrobial]] and [[antiviral]] properties, it is widely used in wound and burn treatments approved by the U.S. [[Food and Drug Administration]]. Conversely, it is also used as a bacterial culture medium.<ref>{{cite journal|url= https://doi.org/10.1016/j.biombioe.2018.07.023|title= Production of medium-chain carboxylic acids by anaerobic fermentation of glycerol using a bioaugmented open culture|journal= Biomass and Bioenergy|year= 2018|doi= 10.1016/j.biombioe.2018.07.023|last1= Dams|first1= Rosemeri I.|last2= Viana|first2= Michael B.|last3= Guilherme|first3= Alexandre A.|last4= Silva|first4= Camila M.|last5= Dos Santos|first5= André B.|last6= Angenent|first6= Largus T.|last7= Santaella|first7= Sandra T.|last8= Leitão|first8= Renato C.|volume= 118|pages= 1–7|s2cid= 106010541|access-date= 16 September 2021|archive-date= 21 February 2022|archive-url= https://web.archive.org/web/20220221143259/https://www.sciencedirect.com/science/article/abs/pii/S0961953418301934?via%3Dihub|url-status= live}}</ref> Its presence in blood can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a [[humectant]] in [[pharmaceutical formulation]]s. Because of its three [[hydroxyl group]]s, glycerol is [[miscible]] with [[water]] and is [[Hygroscopy|hygroscopic]] in nature.<ref name="Ullmann"/>
==Structure==
Line 88 ⟶ 94:
Typical plant sources include [[soybeans]] or [[Arecaceae|palm]]. Animal-derived [[tallow]] is another source. Approximately 950,000 tons per year are produced in the United States and Europe; 350,000 tons of glycerol were produced per year in the U.S. alone from 2000 to 2004.<ref>{{cite web |title= A Glycerin Factor |author= Nilles, Dave |url= https://www.biodieselmagazine.com/article.jsp?article_id=377 |publisher= Biodiesel Magazine |year= 2005 |access-date= 21 February 2022 |archive-date= 8 November 2007 |archive-url= https://web.archive.org/web/20071108114027/http://www.biodieselmagazine.com/article.jsp?article_id=377 |url-status= live }}</ref> The [[Directive on the Promotion of the use of biofuels and other renewable fuels for transport|EU directive 2003/30/EC]] set a requirement that 5.75% of petroleum fuels were to be replaced with [[biofuel]] sources across all [[Member state of the European Union|member states]] by 2010. It was projected in 2006 that by 2020, production would be six times more than demand, creating an excess of glycerol as a byproduct of biofuel production.<ref name="Ullmann">{{cite encyclopedia |encyclopedia= Ullmann's Encyclopedia of Industrial Chemistry |last1= Christoph |first1= Ralf |last2= Schmidt |first2= Bernd |last3= Steinberner |first3= Udo |last4= Dilla |first4= Wolfgang |last5= Karinen |first5= Reetta |year= 2006 |doi= 10.1002/14356007.a12_477.pub2 |chapter= Glycerol |isbn= 3527306730}}</ref>
Glycerol from triglycerides is produced on a large scale, but the crude product is of variable quality, with a low selling price of as low as US$0.02–0.05 per kilogram in 2011.<ref>{{cite journal |last1 = San Kong |first1 = Pei |last2 = Kheireddine Aroua |first2 = Mohamed |last3 = Ashri Wan Daud |first3 = Wan Mohd |year = 2016 |title = Conversion of crude and pure glycerol into derivatives: A feasibility evaluation |journal = Renewable and Sustainable Energy Reviews |volume = 63 |pages = 533–555 |doi = 10.1016/j.rser.2016.05.054 |bibcode = 2016RSERv..63..533K }}</ref> It can be purified, but the process is expensive. Some glycerol is burned for energy, but its heat value is low.<ref>{{cite news |url= https://www.biodieselmagazine.com/articles/8137/clearing-the-way-for-byproduct-quality |work= Biodiesel Magazine |title= Clearing the way for byproduct quality: why quality for glycerin is just as important for biodiesel |author= Sims, Bryan |date= 25 October 2011 |access-date= 21 February 2022 |archive-date= 29 April 2021 |archive-url= https://web.archive.org/web/20210429154729/http://www.biodieselmagazine.com/articles/8137/clearing-the-way-for-byproduct-quality |url-status= live}}</ref>
Crude glycerol from the hydrolysis of triglycerides can be purified by treatment with [[activated carbon]] to remove organic impurities, alkali to remove unreacted glycerol esters, and [[ion exchange]] to remove salts. High purity glycerol (greater than 99.5%) is obtained by multi-step distillation; a [[vacuum chamber]] is necessary due to its high boiling point (290 °C).<ref name=Ullmann/>
===Synthetic glycerol===
{{See also|#Chemical intermediate}}
Although usually not cost-effective, glycerol can be produced by various routes from [[propene]]. The epichlorohydrin process is the most important: it involves the [[Chlorination reaction|chlorination]] of propylene to give [[allyl chloride]], which is oxidized with [[hypochlorite]] to [[dichlorohydrin]], which reacts with a strong base to give [[epichlorohydrin]]. Epichlorohydrin can be hydrolyzed to glycerol. [[Chlorine]]-free processes from propylene include the synthesis of glycerol from [[acrolein]] and [[propylene oxide]].<ref name="Ullmann" />▼
:[[File:Synthetic routes to glycerol.png|600px]]▼
Because of the large-scale production of [[biodiesel]] from fats, where glycerol is a waste product, the market for glycerol is depressed. Thus, synthetic processes are not [[economical]]. Owing to oversupply, efforts are being made to convert glycerol to synthetic precursors, such as [[acrolein]] and epichlorohydrin.<ref>{{cite journal |title= Glycerol|last= Yu|first= Bin|journal= Synlett|volume= 25|issue= 4|pages= 601–602|doi= 10.1055/s-0033-1340636|year= 2014|doi-access= free}}</ref>
▲Although usually not cost-effective because so much is produced from processing of fats, glycerol can be produced by various routes. from During [[propeneWorld War II]]., Thesynthetic epichlorohydringlycerol processprocesses isbecame thea mostnational important:defense priorities because it involvesis a precursor to [[nitroglycerine]]. Epichlorohydrin is the most important precursor. [[Chlorination reaction|chlorination]] of propylene to givegives [[allyl chloride]], which is oxidized with [[hypochlorite]] to [[dichlorohydrin]], which reacts with a strong base to give [[epichlorohydrin]]. Epichlorohydrin can be hydrolyzed to glycerol. [[Chlorine]]-free processes from propylene include the synthesis of glycerol from [[acrolein]] and [[propylene oxide]].<ref name="Ullmann" />
▲: [[File:Synthetic routes to glycerol.png|600px]]
==Applications==
Line 106 ⟶ 111:
It is also recommended as an additive when using polyol sweeteners such as [[erythritol]] and [[xylitol]] which have a cooling effect, due to its heating effect in the mouth, if the cooling effect is not wanted.<ref>{{cite web |title= Functional Food Design Rules |author= Nikolov, Ivan |date= 20 April 2014 |url= https://www.ivannikolov.com/functional-food-design-rules-part-2/ |access-date= 21 February 2022 |archive-date= 30 April 2021 |archive-url= https://web.archive.org/web/20210430030741/https://ivannikolov.com/functional-food-design-rules-part-2/ |url-status= live }}</ref>
Excessive consumption by children can lead to glycerol intoxication.<ref>{{Cite web |last=Burrell |first=Chloe |date=2023-06-02 |title=Perth and Kinross parents warned as 'intoxicated' kids hospitalised by slushy drinks |url=https://www.thecourier.co.uk/fp/news/perth-kinross/4445065/perth-kinross-warning-slushy-drinks/ |access-date=2023-06-03 |website=The Courier |language=en-GB}}</ref> Symptoms of intoxication include [[hypoglycemia]], [[nausea]] and a loss of consciousness ([[Syncope (medicine)|syncope]]). While intoxication as a result of excessive glycerol consumption is rare and its symptoms generally mild, occasional reports of hospitalization have occurred. In the United Kingdom in August 2023, manufacturers of syrup used in slush ice drinks were advised to reduce the amount of glycerol in their formulations by the Food Standards Agency to reduce the risk of intoxication.<ref>{{Cite web |title=‘Not suitable for under-4s’: New industry guidance issued on glycerol in slush-ice drinks |url=https://www.food.gov.uk/news-alerts/news/not-suitable-for-under-4s-new-industry-guidance-issued-on-glycerol-in-slush-ice-drinks |access-date=2023-08-11 |website=Food Standards Agency |language=en}}</ref> ▼
===Medical, pharmaceutical and personal care applications===
Line 114 ⟶ 117:
[[File:Dollop of hair gel.jpg|thumb|Glycerol is an ingredient in products such as hair gel]]
[[File:Glycerin suppositories.jpg|thumb|right|Glycerol suppositories used as laxatives]]
Glycerin is mildly antimicrobial and antiviral and is an FDA-approved treatment for wounds. The Red Cross reports that an 85% solution of glycerin shows bactericidal and antiviral effects, and wounds treated with glycerin show reduced inflammation after roughly two hours. Due to this it is used widely in wound care products, including glycerin based [[Hydrogel dressing|hydrogel]] sheets for burns and other wound care. It is approved for all types of wound care except third-degree burns, and is used to package donor skin used in skin grafts.<ref>{{cite journal |last1=Stout |first1=Edward I. |last2=McKessor |first2=Angie |title=Glycerin-Based Hydrogel for Infection Control |journal=Advances in Wound Care |date=February 2012 |volume=1 |issue=1 |pages=48–51 |doi=10.1089/wound.2011.0288 |pmid=24527279 |pmc=3839013 }}</ref>
Glycerol is used in [[medicine|medical]], [[pharmaceutical]] and [[personal care]] preparations, often as a means of improving smoothness, providing [[lubrication]], and as a [[humectant]].
[[Ichthyosis]] and [[xerosis]] have been relieved by the topical use of glycerin.<ref>{{cite book|title=Ichthyosis: New Insights for the Healthcare Professional|date=22 July 2013|publisher=[[ScholarlyEditions]]|language=en|isbn=9781481659666|page=22}}</ref><ref name="LebwohlHeymann2017">{{cite book|title=Treatment of Skin Disease E-Book: Comprehensive Therapeutic Strategies|date=19 September 2017|publisher=Elsevier Health Sciences|language=en|isbn=9780702069130 |author=Mark G. Lebwohl |author2=Warren R. Heymann |author3=John Berth-Jones |author4=Ian Coulson}}</ref> It is found in allergen [[immunotherapies]], [[cough syrup]]s, [[elixir]]s and [[expectorant]]s, [[toothpaste]], [[mouthwash]]es, [[skin care]] products, shaving cream, [[hair care]] products, [[soap]]s, and water-based [[personal lubricant]]s. In solid dosage forms like tablets, glycerol is used as a tablet holding agent. For human consumption, glycerol is classified by the FDA among the [[sugar alcohol]]s as a caloric macronutrient. Glycerol is also used in [[blood banking]] to preserve [[red blood cell]]s prior to freezing.{{cn|date=April 2024}}
Glycerol is a component of [[glycerin soap]].<!-- PLEASE DO NOT try to enumerate the ingredients for glycerine soap. There are many differing recipes, each with its own "purist" contingent that swears they are the one true glycerine soapmaker --> [[Essential oil]]s are added for [[fragrance]]. This kind of soap is used by people with sensitive, easily irritated [[skin]] because it prevents skin dryness with its [[moisturizer|moisturizing]] properties. It draws moisture up through skin layers and slows or prevents excessive drying and evaporation.{{citation needed|date=February 2013}}
Taken rectally, glycerol functions as a [[laxative]] by irritating the anal mucosa and inducing a [[Osmotic laxative|hyperosmotic effect]],<ref>{{cite web |url= https://www.drugs.com/cdi/glycerin-enema.html |title= Glycerin Enema |publisher= Drugs.com |access-date= 17 November 2012 |archive-date= 6 November 2012 |archive-url= https://web.archive.org/web/20121106235146/http://www.drugs.com/cdi/glycerin-enema.html |url-status= live }}</ref> expanding the [[Large intestine|colon]] by drawing water into it to induce [[peristalsis]] resulting in [[Defecation|evacuation]].<ref>{{cite web |url=https://www.cancer.gov/publications/dictionaries/cancer-drug/def/glycerin-enema |title=glycerin enema |work=NCI Drug Dictionary |publisher=[[National Cancer Institute]] |access-date=2019-05-02 |date=2 February 2011 |archive-date=2 May 2019 |archive-url=https://web.archive.org/web/20190502220442/https://www.cancer.gov/publications/dictionaries/cancer-drug/def/glycerin-enema |url-status=live }}</ref> It may be administered undiluted either as a [[suppository]] or as a small-volume (2–10 ml) [[enema]]. Alternatively, it may be administered in a dilute solution, such as 5%, as a high-volume enema.<ref>{{Citation |author=E. Bertani |author2=A. Chiappa |author3=R. Biffi |author4=P. P. Bianchi |author5=D. Radice |author6=V. Branchi |author7=S. Spampatti |author8=I. Vetrano |author9=B. Andreoni | title = Comparison of oral polyethylene glycol plus a large volume glycerine enema with a large volume glycerine enema alone in patients undergoing colorectal surgery for malignancy: a randomized clinical trial | journal = Colorectal Disease | volume = 13 | issue = 10 | pages = e327–e334 | year = 2011 | pmid = 21689356 | doi = 10.1111/j.1463-1318.2011.02689.x | s2cid = 32872781}}</ref>
Line 144 ⟶ 143:
Like [[ethylene glycol]] and propylene glycol, glycerol is a non-ionic [[kosmotropic|kosmotrope]] that forms strong hydrogen bonds with water molecules, competing with water-water [[hydrogen bonds]]. This interaction disrupts the formation of ice. The minimum freezing point temperature is about {{convert|−36|F|C|order=flip}} corresponding to 70% glycerol in water.
Glycerol was historically used as an anti-freeze for automotive applications before being replaced by ethylene glycol, which has a lower freezing point. While the minimum freezing point of a glycerol-water mixture is higher than an ethylene glycol-water mixture, glycerol is not toxic and is being re-examined for use in automotive applications.<ref>{{cite book |last1= Hudgens |first1= R. Douglas |title= SAE Technical Paper Series |volume= 1 |last2= Hercamp |first2= Richard D. |last3= Francis |first3= Jaime |last4= Nyman |first4= Dan A. |last5= Bartoli |first5= Yolanda |year= 2007 |doi= 10.4271/2007-01-4000 |chapter= An Evaluation of Glycerin (Glycerol) as a Heavy Duty Engine Antifreeze/Coolant Base |series= SAE Technical Paper Series}}</ref><ref>[https://www.astmnewsroom.org/default.aspx?pageid=2115&year=2010&category=Standards%2FTechnical Proposed ASTM Engine Coolant Standards Focus on Glycerin] {{Webarchive|url=https://web.archive.org/web/20170914125754/http://www.astmnewsroom.org/default.aspx?pageid=2115&year=2010&category=Standards%2FTechnical |date=14 September 2017}}. Astmnewsroom.org. Retrieved on 15 August 2012</ref>
In the laboratory, glycerol is a common component of solvents for [[enzymatic]] [[reagents]] stored at temperatures below {{convert|0|C|F}} due to the [[Freezing-point depression|depression of the freezing temperature]]. It is also used as a [[cryoprotectant]] where the glycerol is dissolved in water to reduce damage by ice crystals to laboratory organisms that are stored in frozen solutions, such as [[fungi]], [[bacteria]], [[nematode]]s, and mammalian embryos. Some organisms like the [[moor frog]] produce glycerol to survive freezing temperatures during hibernation.<ref name=":16Shekhovtsov-2022">{{Cite journal |last1=Shekhovtsov |first1=Sergei V. |last2=Bulakhova |first2=Nina A. |last3=Tsentalovich |first3=Yuri P. |last4=Zelentsova |first4=Ekaterina A. |last5=Meshcheryakova |first5=Ekaterina N. |last6=Poluboyarova |first6=Tatiana V. |last7=Berman |first7=Daniil I. |date=January 2022 |title=Metabolomic Analysis Reveals That the Moor Frog ''Rana arvalis'' Uses Both Glucose and Glycerol as Cryoprotectants |journal=Animals |language=en |volume=12 |issue=10 |pages=1286 |doi=10.3390/ani12101286 |issn=2076-2615 |pmc=9137551 |pmid=35625132 |doi-access=free}}</ref>
===Chemical intermediate===
Glycerol is used to produce a variety of useful derivatives.
Glycerol is used to produce [[nitroglycerin]], which is an essential ingredient of various explosives such as [[dynamite]], [[gelignite]], and propellants like [[cordite]]. Reliance on soap-making to supply co-product glycerol made it difficult to increase production to meet wartime demand. Hence, synthetic glycerol processes were national defense priorities in the days leading up to World War II. Nitroglycerin, also known as glyceryl trinitrate (GTN) is commonly used to relieve [[angina pectoris]], taken in the form of [[sub-lingual]] tablets, patches, or as an [[aerosol]] spray.▼
▲Glycerol is used to[[Nitration]] producegives [[nitroglycerin]], which is an essential ingredient of various explosives such as [[dynamite]], [[gelignite]], and propellants like [[cordite]]. RelianceNitroglycerin on soap-making to supply co-product glycerol made it difficult to increase production to meet wartime demand. Hence, synthetic glycerol processes were national defense priorities inunder the days leading up to World War II. Nitroglycerin, also known asname glyceryl trinitrate (GTN) is commonly used to relieve [[angina pectoris]], taken in the form of [[sub-lingual]] tablets, patches, or as an [[aerosol]] spray.
Trifunctional [[polyether polyol]]s are produced from glycerol and [[propylene oxide]]. An oxidation of glycerol affords [[mesoxalic acid]].<ref name="ciri2">{{cite journal |last1 = Ciriminna |first1 = Rosaria |last2 = Pagliaro |first2 = Mario |year = 2003 |title = One-Pot Homogeneous and Heterogeneous Oxidation of Glycerol to Ketomalonic Acid Mediated by TEMPO |journal = Advanced Synthesis & Catalysis |volume = 345 |issue = 3 |pages = 383–388 |doi = 10.1002/adsc.200390043}}</ref> Dehydrating glycerol affords [[hydroxyacetone]].▼
Trifunctional [[polyether polyol]]s are produced from glycerol and [[propylene oxide]].
▲Trifunctional [[polyether polyol]]s are produced from glycerol and [[propylene oxide]]. An oxidationOxidation of glycerol affords [[mesoxalic acid]].<ref name="ciri2">{{cite journal |last1 = Ciriminna |first1 = Rosaria |last2 = Pagliaro |first2 = Mario |year = 2003 |title = One-Pot Homogeneous and Heterogeneous Oxidation of Glycerol to Ketomalonic Acid Mediated by TEMPO |journal = Advanced Synthesis & Catalysis |volume = 345 |issue = 3 |pages = 383–388 |doi = 10.1002/adsc.200390043}}</ref> Dehydrating glycerol affords [[hydroxyacetone]].
Chlorination of glycerol gives the [[1-chloropropane-2,3-diol]]:
: {{chem2|HOCH(CH2OH)2 + HCl -> HOCH(CH2Cl)(CH2OH) + H2O}}
The same compound can be produced by hydrolysis of epichlorohydrin.<ref>{{cite journal |doi=10.1021/cr5004002 |title=Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies |date=2015 |last1=Sutter |first1=Marc |last2=Silva |first2=Eric Da |last3=Duguet |first3=Nicolas |last4=Raoul |first4=Yann |last5=Métay |first5=Estelle |last6=Lemaire |first6=Marc |journal=Chemical Reviews |volume=115 |issue=16 |pages=8609–8651 |pmid=26196761 |url=https://hal.archives-ouvertes.fr/hal-01312971/file/ChemRev_2015_115_8609-8651.pdf }}</ref>
===Vibration damping===
Line 169 ⟶ 176:
===Research on additional uses===
Research continues into potential [[value-added]] products of glycerol obtained from biodiesel production.<ref>{{cite journal |doi= 10.1002/ep.10225 |title= The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production |year= 2007 |last1= Johnson |first1= Duane T. |last2= Taconi |first2= Katherine A. |journal= Environmental Progress |volume= 26 |issue= 4 |pages= 338–348|bibcode= 2007EnvPr..26..338J }}</ref> Examples (aside from combustion of waste glycerol):
* [[Hydrogen]] [[gas]] production.<ref>{{cite journal |author1= Marshall, A. T. |author2 =Haverkamp, R. G. |title= Production of hydrogen by the electrochemical reforming of glycerol-water solutions in a PEM electrolysis cell |year= 2008 |journal= [[International Journal of Hydrogen Energy]] |volume= 33 |issue= 17 |pages= 4649–4654 |doi= 10.1016/j.ijhydene.2008.05.029|bibcode =2008IJHE...33.4649M }}</ref>
* [[Glycerine acetate]] is a potential fuel additive.<ref>{{cite journal |title= Acidic mesoporous silica for the acetylation of glycerol: Synthesis of bioadditives to petrol fuel |year= 2007 |journal= [[Energy & Fuels]] |volume= 21 |issue= 3 |pages= 1782–1791 |doi= 10.1021/ef060647q |last1= Melero |first1= Juan A. |last2= Van Grieken |first2= Rafael |last3= Morales |first3= Gabriel |last4= Paniagua |first4= Marta}}</ref>
* Additive for [[starch]] thermoplastic.<ref>{{Cite journal|last1=Özeren|first1=Hüsamettin D.|last2=Olsson|first2=Richard T.|last3=Nilsson|first3=Fritjof|last4=Hedenqvist|first4=Mikael S. |date=2020-02-01|title=Prediction of plasticization in a real biopolymer system (starch) using molecular dynamics simulations |journal=Materials & Design |language=en |volume=187|pages=108387|doi=10.1016/j.matdes.2019.108387|issn=0264-1275|doi-access=free}}</ref><ref>{{Cite journal|last1=Özeren|first1=Hüsamettin Deniz|last2=Guivier|first2=Manon|last3=Olsson|first3=Richard T.|last4=Nilsson|first4=Fritjof|last5=Hedenqvist|first5=Mikael S. |date=2020-04-07|title=Ranking Plasticizers for Polymers with Atomistic Simulations; PVT, Mechanical Properties and the Role of Hydrogen Bonding in Thermoplastic Starch |journal=ACS Applied Polymer Materials |volume=2|issue=5|pages=2016–2026|doi=10.1021/acsapm.0c00191|doi-access=free}}</ref>
* Conversion to various other chemicals:
** [[Propylene glycol]]<ref>{{cite press release |title= Dow achieves another major milestone in its quest for sustainable chemistries |date= 15 March 2007 |publisher= [[Dow Chemical Company]] |url= https://www.dow.com/propyleneglycol/news/20070315b.htm |access-date= 13 July 2007 |archive-url= https://web.archive.org/web/20090916061724/https://www.dow.com/propyleneglycol/news/20070315b.htm |archive-date= 16 September 2009 |url-status= dead }}</ref>
** [[Acrolein]]<ref>{{cite journal |doi= 10.1039/b506285c |title= The catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production |journal= [[Green Chemistry (journal)|Green Chemistry]] |volume= 8 |issue= 2 |pages= 214–220 |year= 2006 |last1= Ott |first1= L. |last2= Bicker |first2= M. |last3= Vogel |first3= H.}}</ref><ref>{{cite journal |title= Acrolein synthesis from glycerol in hot-compressed water |journal= [[Bioresource Technology]] |year= 2007 |volume= 98 |pages= 1285–1290 |doi= 10.1016/j.biortech.2006.05.007 |pmid= 16797980 |issue= 6 |last1= Watanabe |first1= Masaru |last2= Iida |first2= Toru |last3= Aizawa |first3= Yuichi |last4= Aida |first4= Taku M. |last5= Inomata |first5= Hiroshi|bibcode= 2007BiTec..98.1285W }}</ref><ref>{{Cite journal |last1=Abdullah |first1=Anas |last2=Zuhairi Abdullah |first2=Ahmad |last3=Ahmed |first3=Mukhtar |last4=Khan |first4=Junaid |last5=Shahadat |first5=Mohammad |last6=Umar |first6=Khalid |last7=Alim |first7=Md Abdul |date=March 2022 |title=A review on recent developments and progress in sustainable acrolein production through catalytic dehydration of bio-renewable glycerol |url=https://linkinghub.elsevier.com/retrieve/pii/S0959652622005145 |journal=Journal of Cleaner Production |language=en |volume=341 |pages=130876 |doi=10.1016/j.jclepro.2022.130876|bibcode=2022JCPro.34130876A |s2cid=246853148 }}</ref>
** [[Ethanol]]<ref name="Yazdani">{{cite journal |author1= Yazdani, S. S. |author2 =Gonzalez, R. |title= Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry |year= 2007 |journal= [[Current Opinion in Biotechnology]] |volume= 18 |issue= 3 |pages= 213–219 |doi= 10.1016/j.copbio.2007.05.002 |pmid= 17532205}}</ref><ref>{{cite press release |date=27 June 2007 |title=Engineers Find Way To Make Ethanol, Valuable Chemicals From Waste Glycerin |website=ScienceDaily |url=https://www.sciencedaily.com/releases/2007/06/070626115246.htm}}</ref>
** [[Epichlorohydrin]],<ref>{{cite press release |publisher= [[Dow Chemical Company]] |url= https://epoxy.dow.com/epoxy/news/2007/20070326b.htm |title= Dow Epoxy advances glycerine-to-epichlorohydrin and liquid epoxy resins projects by choosing Shanghai site |date= 26 March 2007 |access-date= 21 February 2022 |archive-date= 8 December 2011 |archive-url= https://web.archive.org/web/20111208095306/http://epoxy.dow.com/epoxy/news/2007/20070326b.htm |url-status= live }}</ref> a raw material for [[epoxy resins]]
Line 199 ⟶ 206:
</div>
The enzyme [[glycerol kinase]] is present mainly in the liver and kidneys, but also in other body tissues, including muscle and brain.<ref>{{cite journal |pmc=1163884|pmid=183753|year=1976|last1=Tildon|first1=J. T.|title=Mitochondrial glycerol kinase activity in rat brain|journal=The Biochemical Journal|volume=157|issue=2|pages=513–516|last2=Stevenson|first2=J. H. Jr.|last3=Ozand|first3=P. T.|doi=10.1042/bj1570513}}</ref><ref>{{cite journal |title=Glycerol kinase activities in muscles from vertebrates and invertebrates|pmid=5801671 |pmc= 1187734 |volume=112|issue=4|date=May 1969|journal=Biochem. J.|pages=465–474|last1=Newsholme|first1=E. A.|last2=Taylor|first2=K|doi=10.1042/bj1120465}}</ref><ref>{{cite journal |vauthors= Jenkins, BT, Hajra, AK |date= 1976 |title= Glycerol Kinase and Dihydroxyacetone Kinase in Rat Brain |journal= Journal of Neurochemistry |volume= 26 |issue= 2 |pages= 377–385 |doi= 10.1111/j.1471-4159.1976.tb04491.x |pmid= 3631 |hdl= 2027.42/65297 |s2cid= 14965948 |url= https://deepblue.lib.umich.edu/bitstream/2027.42/65297/1/j.1471-4159.1976.tb04491.x.pdf |hdl-access= free |access-date= 27 August 2019 |archive-date= 21 February 2022 |archive-url= https://web.archive.org/web/20220221143240/https://deepblue.lib.umich.edu/bitstream/handle/2027.42/65297/j.1471-4159.1976.tb04491.x.pdf;jsessionid=27E5DB761F19C9F26C4F8EF4EDC33819?sequence=1 |url-status= live }}</ref> In adipose tissue, glycerol 3-phosphate is obtained from [[dihydroxyacetone phosphate]] with the enzyme [[glycerol-3-phosphate dehydrogenase]].
==Toxicity and safety==
Glycerol has very low toxicity when ingested; its [[LD50|LD<sub>50</sub>]] oral dose for rats is 12600 mg/kg and 8700 mg/kg for mice. It does not appear to cause toxicity when inhaled, although changes in cell maturity occurred in small sections of lung in animals under the highest dose measured. A sub-chronic 90-day nose-only inhalation study in Sprague–Dawley (SD) rats exposed to 0.03, 0.16 and 0.66 mg/L glycerin (Per liter of air) for 6-hour continuous sessions revealed no treatment-related toxicity other than minimal [[metaplasia]] of the [[epithelium]] lining at the base of the [[epiglottis]] in rats exposed to 0.66 mg/L glycerin.<ref>{{cite journal | year = 2017 | title = Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague–Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints | journal = Food and Chemical Toxicology | volume = 109 | issue = Pt 1| pages = 315–332 | doi = 10.1016/j.fct.2017.09.001 | last1 = Phillips | first1 = Blaine | last2 = Titz | first2 = Bjoern | last3 = Kogel | first3 = Ulrike | last4 = Sharma | first4 = Danilal | last5 = Leroy | first5 = Patrice | last6 = Xiang | first6 = Yang | last7 = Vuillaume | first7 = Grégory | last8 = Lebrun | first8 = Stefan | last9 = Sciuscio | first9 = Davide | last10 = Ho | first10 = Jenny | last11 = Nury | first11 = Catherine | last12 = Guedj | first12 = Emmanuel | last13 = Elamin | first13 = Ashraf | last14 = Esposito | first14 = Marco | last15 = Krishnan | first15 = Subash | last16 = Schlage | first16 = Walter K. | last17 = Veljkovic | first17 = Emilija | last18 = Ivanov | first18 = Nikolai V. | last19 = Martin | first19 = Florian | last20 = Peitsch | first20 = Manuel C. | last21 = Hoeng | first21 = Julia | last22 = Vanscheeuwijck | first22 = Patrick| pmid = 28882640 | doi-access = free }}</ref><ref>{{cite journal | last1 = Renne | first1 = R. A. | last2 = Wehner | first2 = A. P. | last3 = Greenspan | first3 = B. J. | last4 = Deford | first4 = H. S. | last5 = Ragan | first5 = H. A. | last6 = Westerberg | first6 = R. B. | year = 1992 | title = 2-Week and 13-Week Inhalation Studies of Aerosolized Glycerol in Rats | journal = International Forum for Respiratory Research | volume = 4 | issue = 2| pages = 95–111 | doi = 10.3109/08958379209145307| bibcode = 1992InhTx...4...95R }}</ref>▼
===Glycerol intoxication===
▲Excessive consumption by children can lead to glycerol intoxication.<ref>{{Cite web |last=Burrell |first=Chloe |date=2023-06-02 |title=Perth and Kinross parents warned as 'intoxicated' kids hospitalised by slushy drinks |url=https://www.thecourier.co.uk/fp/news/perth-kinross/4445065/perth-kinross-warning-slushy-drinks/ |access-date=2023-06-03 |website=The Courier |language=en-GB}}</ref> Symptoms of intoxication include [[hypoglycemia]], [[nausea]] and a loss of consciousness ([[Syncope (medicine)|syncopeloss of consciousness]]). While intoxication as a result of excessive glycerol consumption is rare and its symptoms generally mild, occasional reports of hospitalization have occurred.<ref>{{Cite web |title=Toddler 'turned grey and passed out' after drinking Slush Puppie |url=https://www.bbc.com/news/articles/c725pzqzdgno |access-date=2024-07-31|date=2024-07-31 |website=www.bbc.com |language=en-GB}}</ref> In the United Kingdom in August 2023, manufacturers of syrup used in [[slushy|slush ice drinksdrink]]s were advised to reduce the amount of glycerol in their formulations by the Food Standards Agency to reduce the risk of intoxication.<ref>{{Cite web |title=‘Not'Not suitable for under-4s’4s': New industry guidance issued on glycerol in slush-ice drinks |url=https://www.food.gov.uk/news-alerts/news/not-suitable-for-under-4s-new-industry-guidance-issued-on-glycerol-in-slush-ice-drinks |access-date=2023-08-11 |website=Food Standards Agency |language=en}}</ref>
[[Food Standards Scotland]] advises that slush ice drinks containing glycerol should not be given to children under the age of 4, owing to the risk of intoxication. It also recommends that businesses do not use [[free refill]] offers for the drinks in venues where children under the age of 10 are likely to consume them, and that products should be appropriately labelled to inform consumers of the presence of glycerol.<ref>{{Cite web |title=Glycerol in slush ice drinks {{!}} Food Standards Scotland |url=https://www.foodstandards.gov.scot/consumers/food-safety/buying-food-eating-out/glycerol-in-slush-ice-drinks |access-date=2024-08-04 |website=www.foodstandards.gov.scot}}</ref>
▲Glycerol has very low toxicity when ingested; its [[LD50|LD<sub>50</sub>]] oral dose for rats is 12600 mg/kg and 8700 mg/kg for mice. It does not appear to cause toxicity when inhaled, although changes in cell maturity occurred in small sections of lung in animals under the highest dose measured. A sub-chronic 90-day nose-only inhalation study in Sprague–Dawley (SD) rats exposed to 0.03, 0.16 and 0.66 mg/L glycerin (Per liter of air) for 6-hour continuous sessions revealed no treatment-related toxicity other than minimal [[metaplasia]] of the [[epithelium]] lining at the base of the [[epiglottis]] in rats exposed to 0.66 mg/L glycerin.<ref>{{cite journal | year = 2017 | title = Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague–Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints | journal = Food and Chemical Toxicology | volume = 109 | issue = Pt 1| pages = 315–332 | doi = 10.1016/j.fct.2017.09.001 | last1 = Phillips | first1 = Blaine | last2 = Titz | first2 = Bjoern | last3 = Kogel | first3 = Ulrike | last4 = Sharma | first4 = Danilal | last5 = Leroy | first5 = Patrice | last6 = Xiang | first6 = Yang | last7 = Vuillaume | first7 = Grégory | last8 = Lebrun | first8 = Stefan | last9 = Sciuscio | first9 = Davide | last10 = Ho | first10 = Jenny | last11 = Nury | first11 = Catherine | last12 = Guedj | first12 = Emmanuel | last13 = Elamin | first13 = Ashraf | last14 = Esposito | first14 = Marco | last15 = Krishnan | first15 = Subash | last16 = Schlage | first16 = Walter K. | last17 = Veljkovic | first17 = Emilija | last18 = Ivanov | first18 = Nikolai V. | last19 = Martin | first19 = Florian | last20 = Peitsch | first20 = Manuel C. | last21 = Hoeng | first21 = Julia | last22 = Vanscheeuwijck | first22 = Patrick| pmid = 28882640 | doi-access = free }}</ref><ref>{{cite journal | last1 = Renne | first1 = R. A. | last2 = Wehner | first2 = A. P. | last3 = Greenspan | first3 = B. J. | last4 = Deford | first4 = H. S. | last5 = Ragan | first5 = H. A. | last6 = Westerberg | first6 = R. B. | year = 1992 | title = 2-Week and 13-Week Inhalation Studies of Aerosolized Glycerol in Rats | journal = International Forum for Respiratory Research | volume = 4 | issue = 2| pages = 95–111 | doi = 10.3109/08958379209145307}}</ref>
==Historical cases of contamination with diethylene glycol==
On 4 May 2007, the FDA advised all U.S. makers of medicines to test all batches of glycerol for [[diethylene glycol]] contamination.<ref>{{cite web |publisher= U.S. Food and Drug Administration |url= https://www.fda.gov/bbs/topics/NEWS/2007/NEW01628.html |title= FDA Advises Manufacturers to Test Glycerin for Possible Contamination |date= 4 May 2007 |access-date= 8 May 2007 |archive-date= 7 May 2007 |archive-url= https://web.archive.org/web/20070507074219/http://www.fda.gov/bbs/topics/NEWS/2007/NEW01628.html |url-status= live}}</ref> This followed an occurrence of [[Toxic cough syrup|hundreds of fatal poisonings in Panama]] resulting from a falsified import customs declaration by Panamanian import/export firm Aduanas Javier de Gracia Express, S. A. The cheaper diethylene glycol was relabeled as the more expensive glycerol.<ref>{{cite news |author= Walt Bogdanich |date= 6 May 2007 |url= https://www.nytimes.com/2007/05/06/world/06poison.html |title= From China to Panama, a Trail of Poisoned Medicine |work= The New York Times |access-date= 8 May 2007 |author-link= Walt Bogdanich |archive-date= 26 September 2015 |archive-url= https://web.archive.org/web/20150926084342/http://www.nytimes.com/2007/05/06/world/06poison.html |url-status= live }}</ref><ref>{{cite news |date= 20 February 2013 |url= https://www.topmastersinpublichealth.com/10-biggest-medical-scandals-in-history |title= 10 Biggest Medical Scandals in History |access-date= 21 February 2022 |archive-date= 8 January 2022 |archive-url= https://web.archive.org/web/20220108074814/https://www.topmastersinpublichealth.com/10-biggest-medical-scandals-in-history/ |url-status= live }}</ref> Between 1990 and 1998, incidents of DEG poisoning reportedly occurred in Argentina, Bangladesh, India, and Nigeria, and resulted in hundreds of deaths. In 1937, more than one lachundred people died in the United arab emitaresStates after ingesting DEG-contaminated elixir sulfanilamide, a drug used to treat infections.<ref>{{Cite journal|last=Lang|first=Les|date=2007-07-01|title=FDA Issues Statement on Diethylene Glycol and Melamine Food Contamination|url=https://www.gastrojournal.org/article/S0016-5085(07)00995-X/abstract|journal=Gastroenterology|language=en|volume=133|issue=1|pages=5–6|doi=10.1053/j.gastro.2007.05.013|pmid=17631118|issn=0016-5085|access-date=25 December 2020|archive-date=21 February 2022|archive-url=https://web.archive.org/web/20220221143257/https://www.gastrojournal.org/article/S0016-5085%2807%2900995-X/fulltext|url-status=live|doi-access=free}}</ref>
==Etymology==
The origin of the ''gly-'' and ''glu''- prefixes for glycols and sugars is from [[Ancient Greek]] {{lang|grc|γλυκύς}} ''glukus'' which means sweet.<ref>[https://www.dictionary.com/browse/glyco- glyco-] {{Webarchive|url=https://web.archive.org/web/20210430152747/https://www.dictionary.com/browse/glyco- |date=30 April 2021}}, dictionary.com</ref> Name ''glycérine'' was coined ca. 1811 by [[Michel Eugène Chevreul]] to denote what was previously called "sweet principle of fat" by its discoverer [[Carl Wilhelm Scheele]]. It was borrowed into English ca. 1838 and in the 20th c. displaced by 1872 term glycerol featuring an alcohols' suffix -ol.
==Properties==
Table of thermal and physical properties of saturated liquid glycerin:<ref>{{Cite book |last=Holman |first=Jack P. |title=Heat Transfer |publisher=McGraw-Hill Companies, Inc. |year=2002 |isbn=9780072406559 |edition=9th |location=New York, NY |pages=600–606 |language=English}}</ref><ref>{{Cite book |last=Incropera 1 Dewitt 2 Bergman 3 Lavigne 4 |first=Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 |title=Fundamentals of Heat and Mass Transfer |publisher=John Wiley and Sons, Inc. |year=2007 |isbn=9780471457282 |edition=6th |location=Hoboken, NJ |pages=941–950 |language=English}}</ref>
: {|class="wikitable mw-collapsible mw-collapsed"
!Temperature (°C)
![[Density]] (kg/m<sup>3</sup>)